Air-Tel e-CRF® Features
Simple and powerful tailor-made E-Crf
A complete set of features for every need
Air-Tel e-CRF® can adapt to your different needs. Thanks to a complete and multipurpose set of features, our E-Crf are your best solution for Electronic Clinical Data Capture in Clinical Studies, Registries and all type of other Clinical projects.
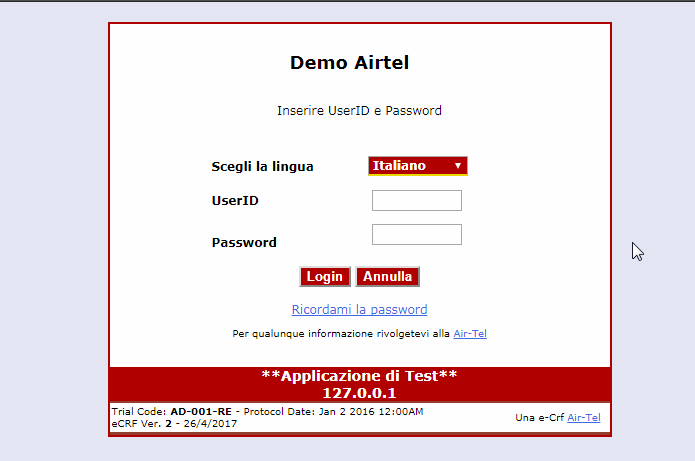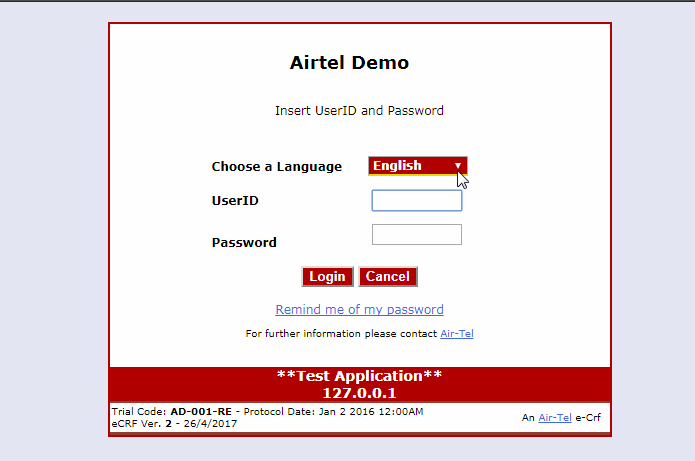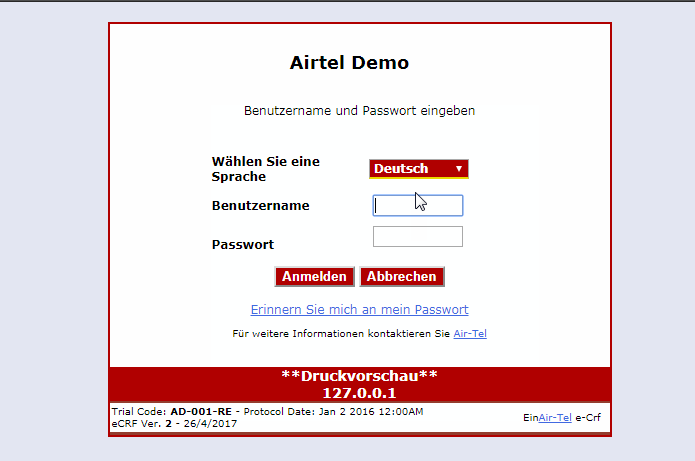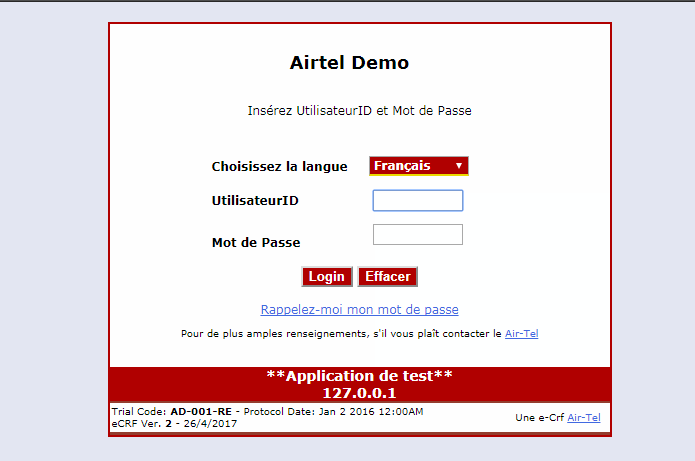
Simple and intuitive interface
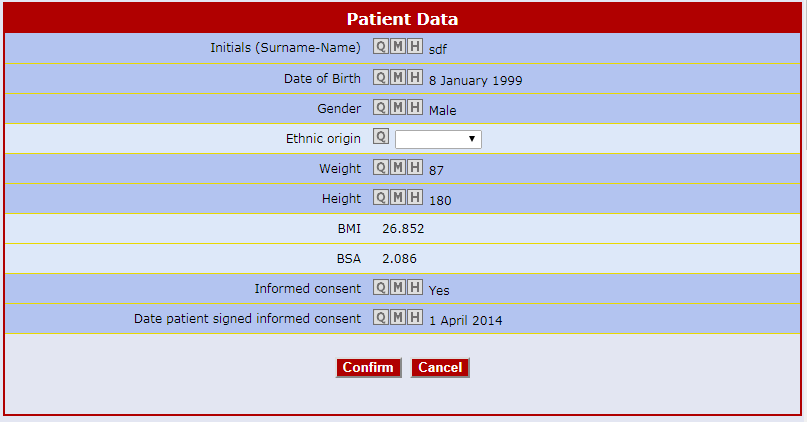
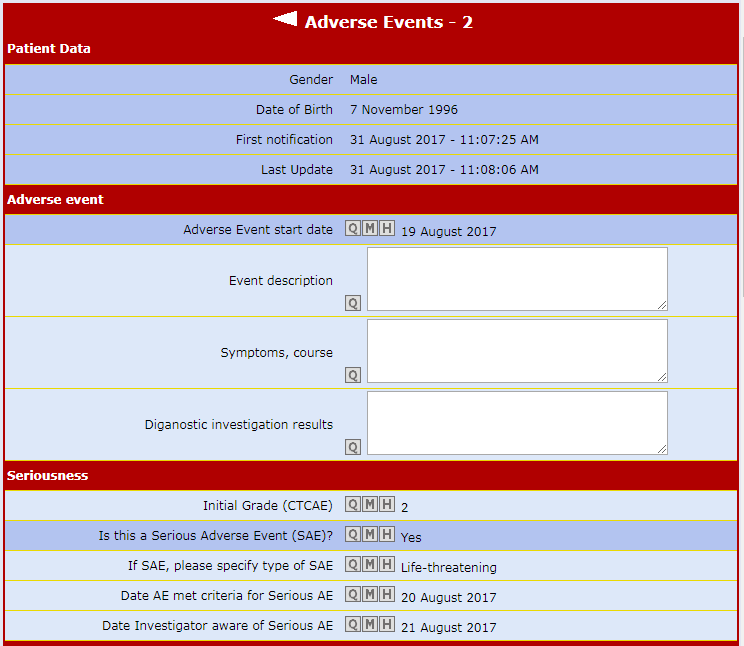
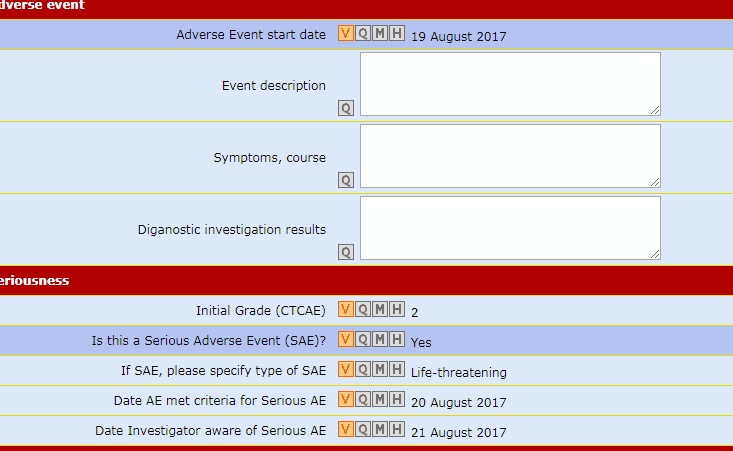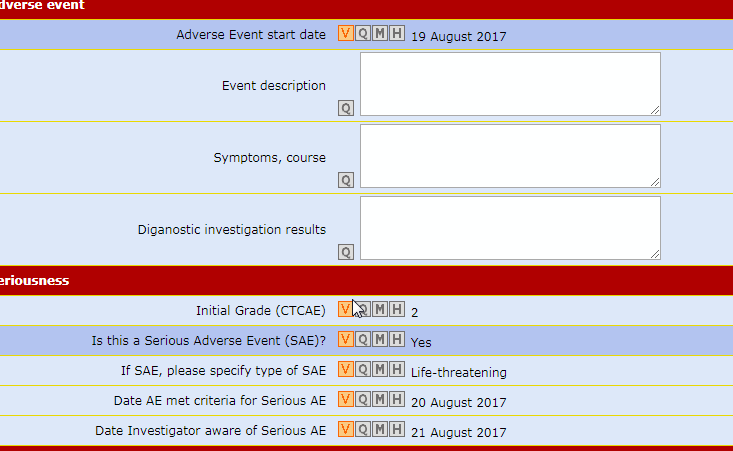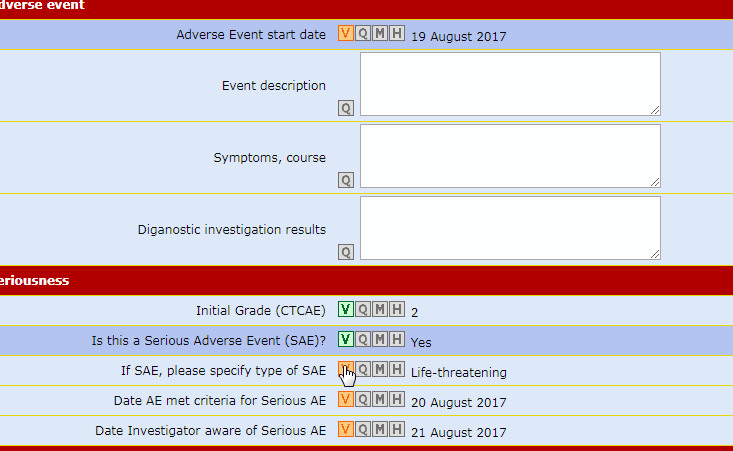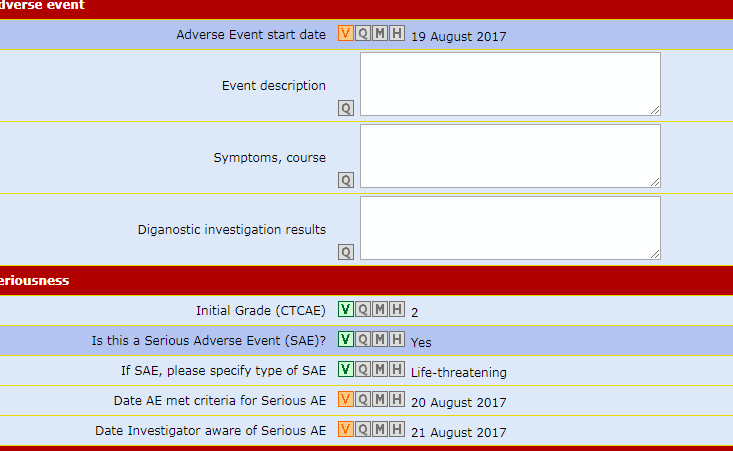
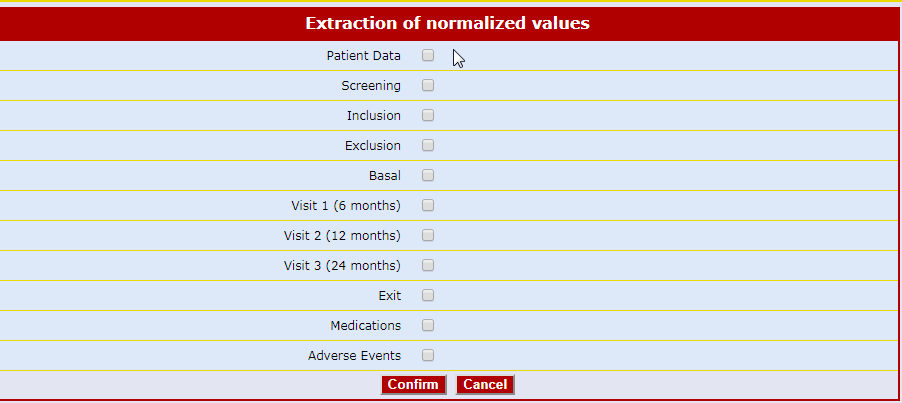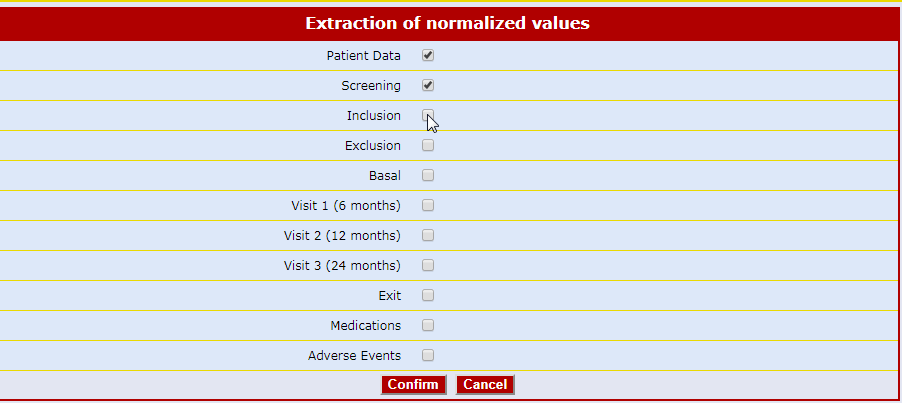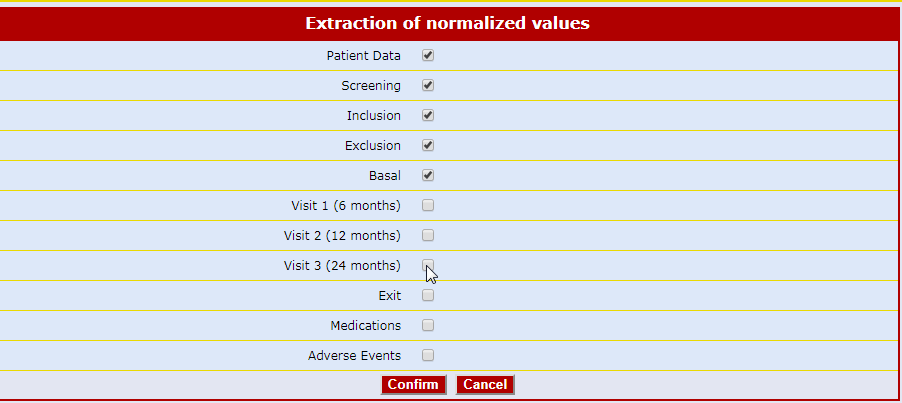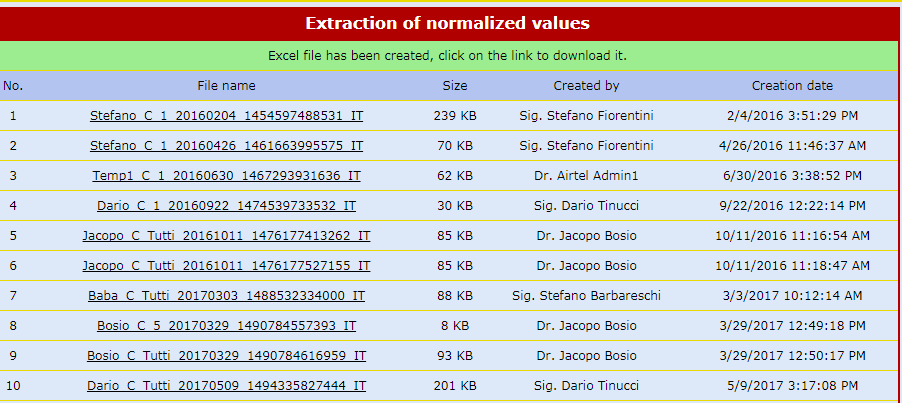
The Air-Tel e-CRF® have a very simple interface: the entry fields of the data are highlighted and ordered in intuitive lists.
All previous data are encapsulated in the background and can be modified in GCP.
Traceability is guaranteed. For data registration, pre-selected codes are used wherever possible (e.g. drop down menus, radio buttons to check) to minimize typing errors.
Guided Fill-in Routes
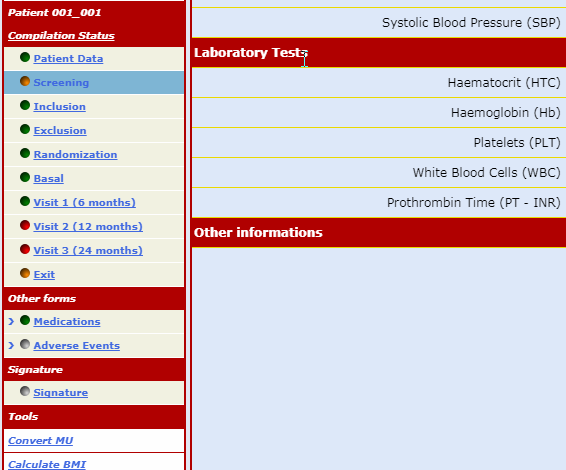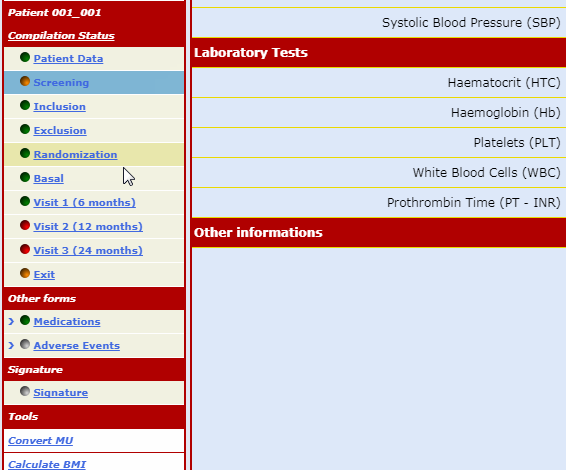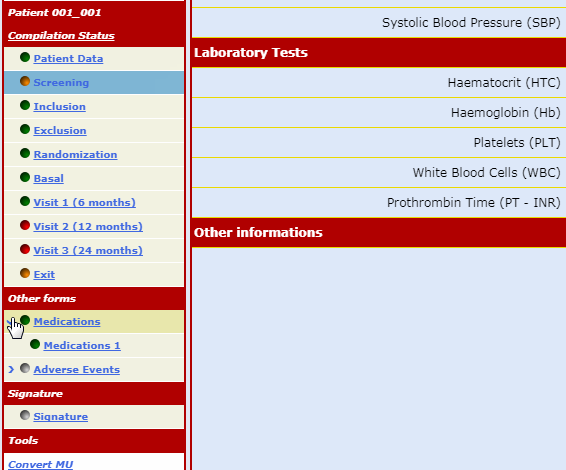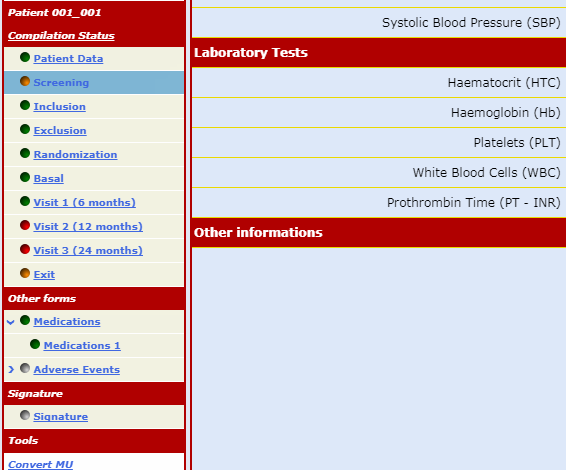
The forms which make up the case report are always visible on the menu bar but can only be opened following a non-intrusive guided fill-in route.
This procedure ensures that any data input finds an exact, unequivocal correspondence reducing the number of consistency checks necessary to 'clean' the database.
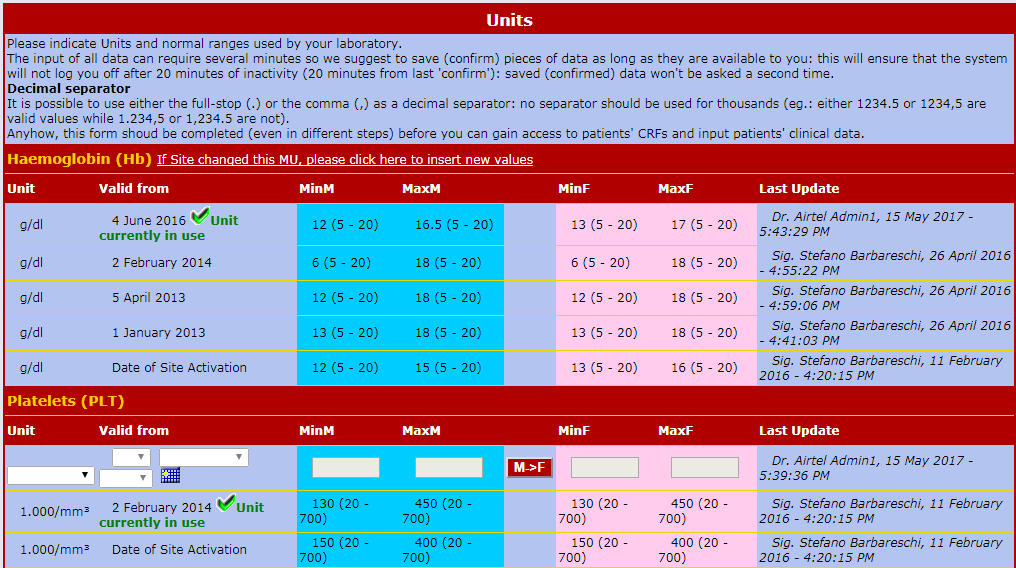
Intelligent Management of the Units of Measurement (UM)
Each Center can use its own UMs (provided by the laboratory) and need to enter it just once.
The internal conversion tables can standardize the data 'on-the-spot' and allow statistical analysis in real-time even if the source Data are expressed in different units.
UMs can change over time. Air-Tel e-CRF® allow each Centre to change its UMs by specifying in which
date interval the UMs are valid. Every update and modifications is tracked, in accordance with GCP.
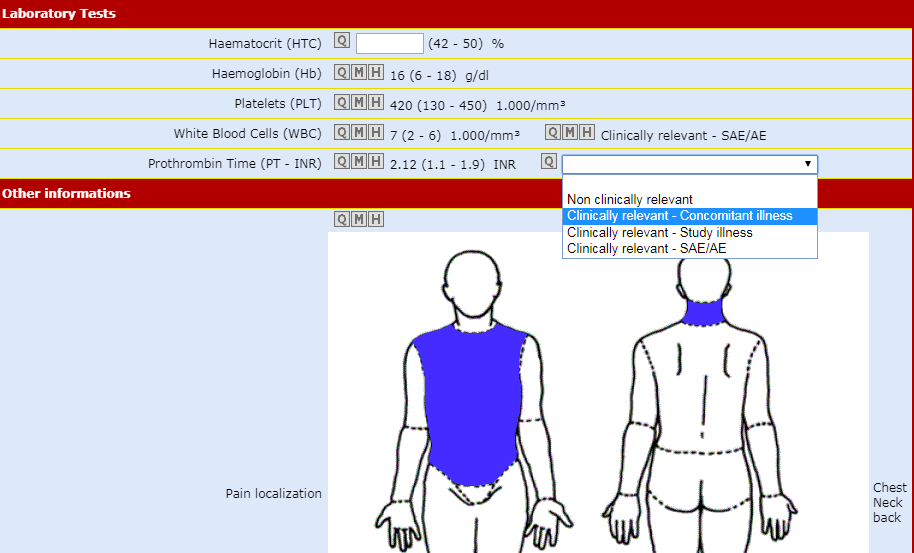
Data Entry Validity Check
Thanks to the UM conversion processes, each piece of lab data entered is automatically compared with its range of plausible values.
Major mistakes are rejected by the system immediately, thus reducing the need to make 'obvious corrections'.
An optional feature of Air-Tel e-CRF® allows to detect in real time data that are inside the range of plausible values but outside the normality range of that Center.
In this case the system automatically presents an additional question to the user, asking him to specify the reason of the "out of range" value thus fulfilling GCP requirements on this matter.
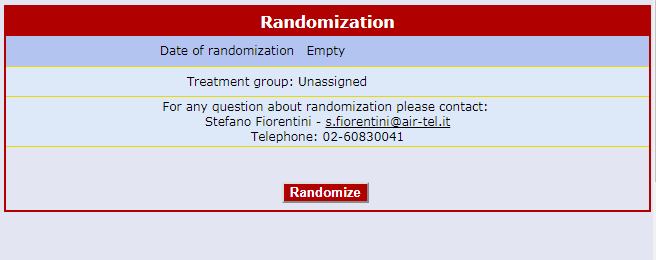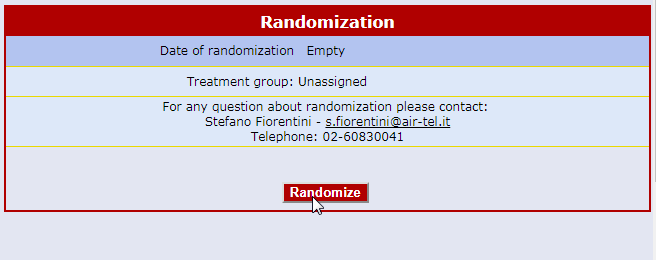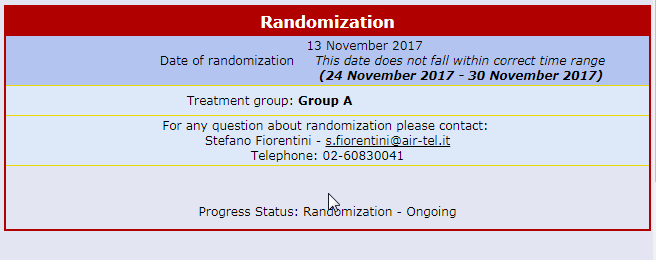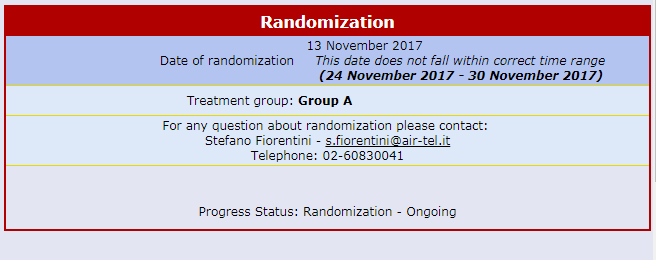
Centralised On-line Randomization
The randomizing software is always available online and connected to each Air-Tel e-CRF®.
When a patient is enrolled, the parameters collected during the screening process are used for the correct allocation, according to the protocol and by using minimization algorithms.
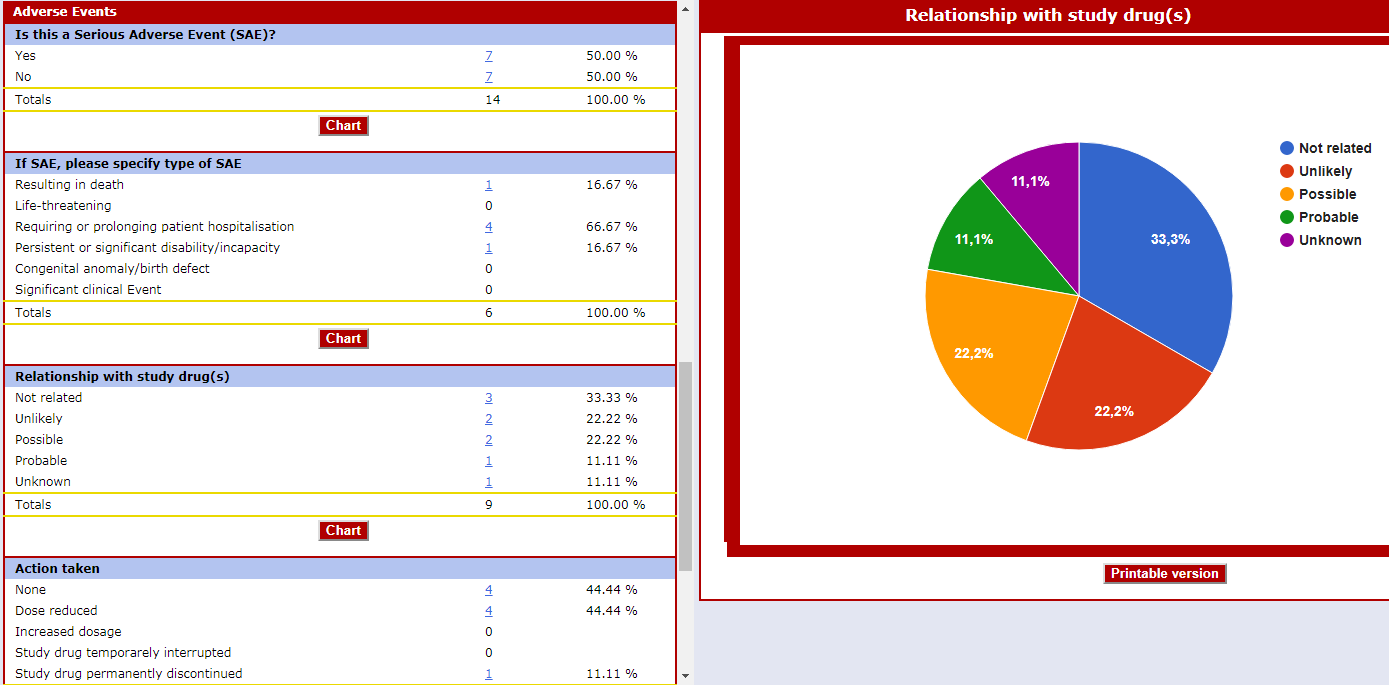
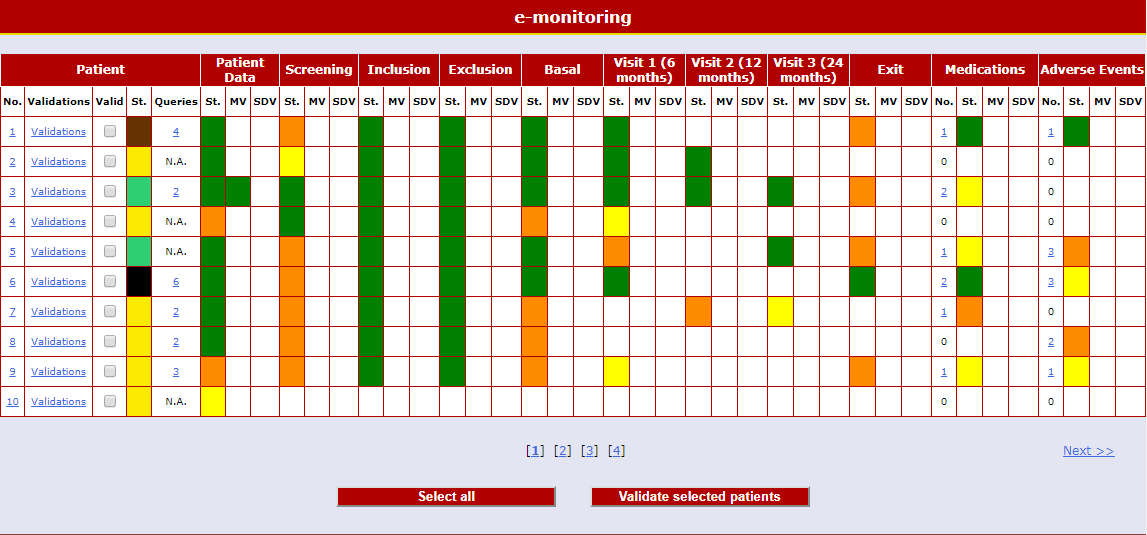
Real-time Statistics by Objectives
Detailed and complete descriptive statistic forms enable the user to navigate the database by objectives rather than by patient.
There are windows related to recruitment, violations, safety, concomitant pathologies, therapies, etc.
Other windows with "Ad hoc" unique statistics are created according to the needs of each clinical study.
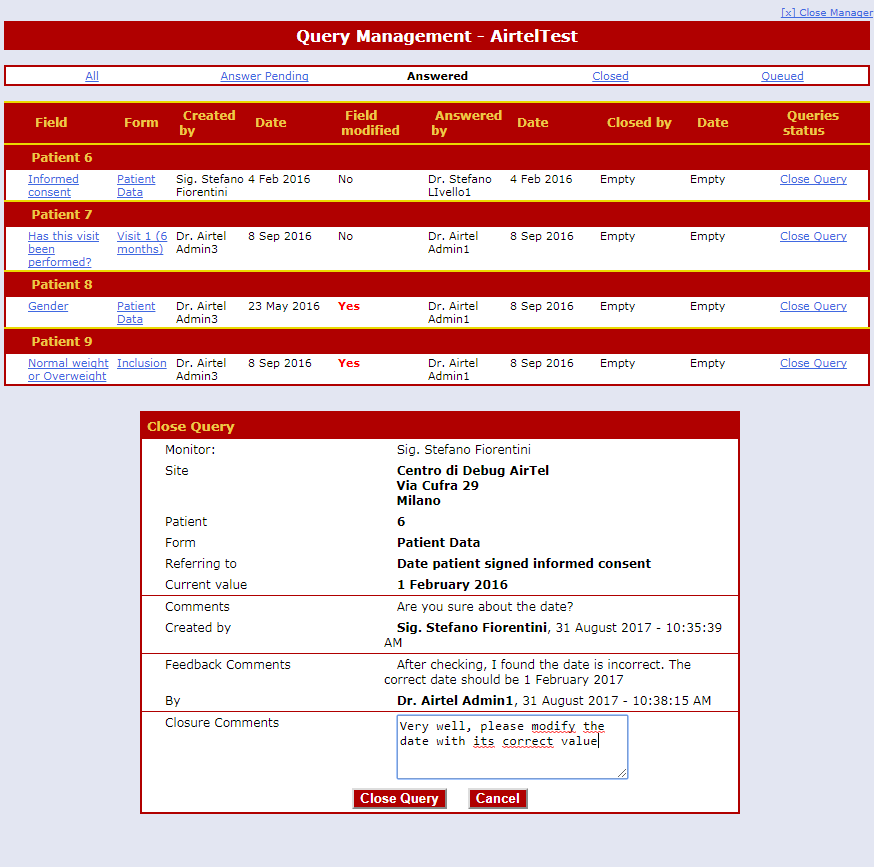
Powerful Query Management System
Any data item can be queried by means of a single click.
All queries are saved in the database and displayed both to the Clinicians and the Monitors.
An optional and flexible email notification system is included.
Queries are answered by the Clinicians and are then closed by the Monitor.
...and many other awesome features we can't wait to show you!